Life sciences companies face pressure to develop new, innovative products rapidly while continually seeking ways to cut design and product costs. Longer life spans and tightening regulations add the further strictures of avoiding litigation and mitigating safety risks. Designers of life sciences products must create profitable product lines quickly enough to harvest their full market value. At the same time, they have to do their utmost to ensure that their products are durable and reliable – minimizing the risk of liability, recalls or regulatory problems.
This article looks at ways that modeling and simulation software can help life sciences product designers accelerate their pace of innovation. Modeling and simulation software enables designers to evaluate a range of design and materials choices before going through the costly process of creating prototypes for physical testing. With this approach, they can move fast and be first to market with products that are adaptable enough to be approved in multiple geographies and reliable enough to mitigate liability risks.
Designing the Coronary Stent, a Tiny Life Saver
To illustrate the potential for modeling and simulation software, we will describe the process of designing and evaluating engineering choices for a coronary stent. Coronary stents are small tube-like structures created from metal mesh. They are inserted into blocked arteries to facilitate blood flow. Though the coiled design of the stent appears simple, the implications of various usage factors make the potential durability and reliability challenges quite extensive. And, the risks are high. These devices are literally sitting in the patient’s blood vessel for the long term. If they malfunction, they can lead to a host of medical difficulties.

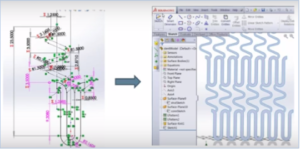
Many variables and usage factors will affect how this design will actually fare once it’s inserted in a patient’s artery. A stent must maintain its shape and perform its artery opening work throughout an average of 500,000 heartbeats a year. Each heartbeat causes pressure on the arterial walls that makes the stent flex. For a 20-year product lifespan, the stent must be able to weather about 10,000,000 compressions and extensions without breaking. That’s a lot of stress on hair-thin slices of stainless steel.
Multiphysics Modeling and Simulation for a Medical Device
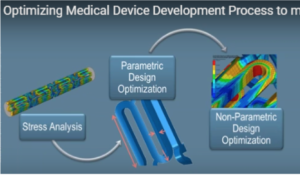
Stress Analysis
Stress analysis involves using fatigue analysis software to assess how much stress the stent’s mesh can withstand at different thicknesses. SIMULIA software enables engineers to model different material choices, e.g. stainless steel versus nitinol, platinum etc., each of which has its own unique stress characteristics and elastic properties. The software can model low-cycle and high-cycle fatigue of the material.
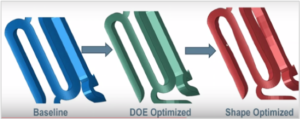
Parametric Design Optimization
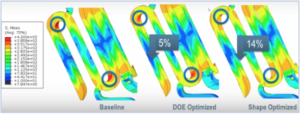
Non-Parametric Design Optimization
Reliability Modeling
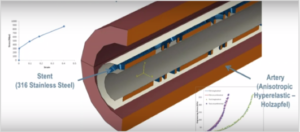
Human bodies vary greatly: People have different arterial wall thicknesses, pulses and blood pressure levels. These three variables can change within the same person over time as well. The stent is not in a static environment. The wrong stent design can lead to poor health outcomes like re-occlusion of the artery or Restenosis, an injuring of the arterial wall. Reliability testing gives the designer a breadth of insight into how the stent will perform across different assumptions about the patient.
Conclusion
The life sciences provide an opportunity to show how modeling and simulation software can facilitate better design when the stakes are high and the physical product is small. In the case of the stent, the goal is to produce a product that has the best chance of performing its therapeutic duty over the long term. The design process has to move quickly but minimize the chance of mechanical failure that could cause health consequences for the patient as well as liability for the manufacturer. On the other end, the product has to be profitable. It has to be easy to manufacture. Modeling and simulation software makes it possible to achieve these goals. With modeling and simulation software, medical device designers can explore multiple design parameters before anything is actually made or implanted in a human body.
Questions? Give Us a Call
3DSMan provides extensive consulting and process assessments on design and simulation processes. Let’s talk and see what your company might be able to do to increase efficiencies.
We’re available at (630) 451-9033, and we’ll be happy to answer your questions. Or feel free to email us.